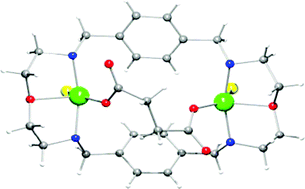
The new dioxatetraazamacrocycle (L1) was synthesized by a 2 + 2 condensation and characterized. Stability constants of its copper(II) complexes were determined by spectrophotometry in DMSO at 298.2 K in 0.10 mol dm−3 KClO4. Mainly dinuclear complexes are formed and the presence of mononuclear species is dependent on the counterion (Cl− or ClO4−). The association constants of the dinuclear copper(II) complexes with dicarboxylate anions [oxalate (oxa2−), malonate (mal2−), succinate (suc2−), and glutarate (glu2−)] were also determined by spectrophotometry at 298.2 K in DMSO, and it was found that values decrease with an increase of the alkyl chain between the carboxylate groups. X-Band EPR spectra of the dicopper(II) complexes and of their cascade species in frozen DMSO exhibit dipole–dipole coupling, and their simulation, together with their UV-vis spectra, showed that the copper centres of the complexes in solution had square pyramidal geometries though with different distortions. From the experimental data, it was also possible to predict the Cu⋯Cu distances, the minimum being found at 6.4 Å for the Cu2L1Cl4 complex and then successively this distance slightly increases when the chloride anions are replaced by dicarboxylate anions, from 6.6 Å for oxa2− to 7.8 Å for glu2−. The crystal structures of the dinuclear copper cascade species with oxa2− and suc2− were determined and showed one anion bridging both copper centres and Cu⋯Cu distances of 5.485(7) Å and 6.442(8) Å, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...