Carbene–pyridine chelating 2Fe2S hydrogenase model complexes as highly active catalysts for the electrochemical reduction of protons from weak acid (HOAc)†
Abstract
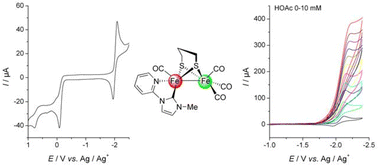
Two asymmetrically disubstituted diiron complexes (µ-pdt)[Fe(CO)3][Fe(CO)(η2-L)] (L = 1-methyl-3-(2-pyridyl)imidazol-2-ylidene (NHCMePy), 2; 1,3-bis(2-picolyl)imidazol-2-ylidene (NHCdiPic), 4) and a mono-substituted diiron complex (µ-pdt)[Fe(CO)3][Fe(CO)2(NHCdiPic)] (3) were prepared as biomimetic models of the Fe-only hydrogenase active site. X-Ray studies show that the NHCMePy and NHCdiPic

 Please wait while we load your content...
Please wait while we load your content...