Modeling the inhibition of peptide deformylase by hydroxamic acids: influence of the sulfur donor
Abstract
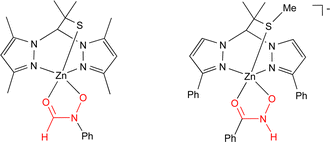
The first complexes containing both a sulfur atom and a hydroxamate moiety coordinated to a biologically relevant transition metal were synthesized as models for the structure of inhibited

 Please wait while we load your content...
Please wait while we load your content...