Insertion reaction of carbon dioxide into Sn–OR bond. Synthesis, structure and DFT calculations of di- and tetranuclear isopropylcarbonato tin(iv) complexes†‡
Abstract
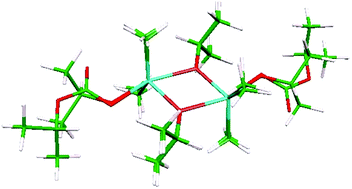
The reaction of carbon dioxide with the stannane nBu2Sn(OiPr)2 and distannoxane [nBu2(iPrO)Sn]2O leads to the selective insertion into one Sn–OiPr bond generating the corresponding nBu2Sn(OiPr)(OCO2iPr) and nBu2(iPrO)SnOSn(OCO2iPr)nBu2 species. Both compounds are characterised by multinuclear
- This article is part of the themed collection: Carbon dioxide at metal centres

 Please wait while we load your content...
Please wait while we load your content...