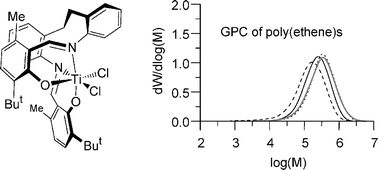
The structural properties of three new classes of titanium and zirconium complex bearing tetradentate salicylaldiminato proligands are elucidated using X-ray diffraction and NMR spectroscopy. On activation with MAO co-catalyst, their behaviour in ethene polymerization depends strongly on the nature of the structure and the substitution pattern. One titanium complex based on a 2-aminobenzylamine (C3-chain) backbone has a trans arrangement of the co-ligand sites and, unsurprisingly, does not polymerize ethene. The 1,8-diaminonaphthalene (C3-chain) backbone gives a rather ring-strained cis complex, but was also unproductive. A range of cis complexes of zirconium with the 2,2′-diaminobibenzyl (C6-chain) backbone give low to moderate productivities of multimodal poly(ethene), while in contrast the structurally analogous titanium compounds provide highly active, single site catalysts. Thermal degradation of these catalysts is slowed significantly by a substitution pattern on the phenolate unit which sterically protects the imine donor unit; a phenomenon which has been previously observed in much lower activity catalysts based on 2,2′-diaminobiphenyl (C4-chain) but which does not improve the stability of the very highly active unbridged systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...