Intramolecular hydroamination/cyclisation of aminoallenes mediated by a cationic zirconocenecatalyst: a computational mechanistic study†
Abstract
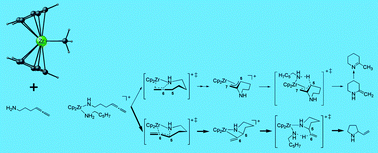
The complete sequence of steps of a tentative catalytic cycle for intramolecular hydroamination/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkage across the Zr–N σ-bond. The alternative exo- and endocyclic pathways show similar probabilities for the sterically less encumbered reactants {1 + 2} investigated herein. However, steric factors are expected to exert control on the regioselectivity of ring closure. On the other hand, the metathesis-type transition states for subsequent protonolysis are indicated to be less sensitive to steric demands. Formation of the six-membered azacycle-Zr intermediate through intramolecular C
C linkage across the Zr–N σ-bond. The alternative exo- and endocyclic pathways show similar probabilities for the sterically less encumbered reactants {1 + 2} investigated herein. However, steric factors are expected to exert control on the regioselectivity of ring closure. On the other hand, the metathesis-type transition states for subsequent protonolysis are indicated to be less sensitive to steric demands. Formation of the six-membered azacycle-Zr intermediate through intramolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C insertion into the Zr–N σ-bond is predicted to be turnover limiting. The factors that govern the regioselectivity of the aminoallene IHC have been elucidated.
C insertion into the Zr–N σ-bond is predicted to be turnover limiting. The factors that govern the regioselectivity of the aminoallene IHC have been elucidated.

 Please wait while we load your content...
Please wait while we load your content...