Synthesis and structural characterisation of lithium and sodium 2,6-dibenzylphenolate complexes
Abstract
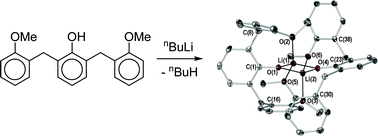
The stoichiometric treatment of 2,6-dibenzylphenol (HOdbp) or 2′,2″-dimethoxy-2,6-dibenzylphenol (HOdbpOMe) with n-butyllithium or sodium bis(trimethylsilyl)amide (the latter as a solution in THF) in Et2O or DME affords the dimeric alkali metal phenolates [{M(Odbp)(L)}2] (M = Li; L = Et2O (1), L = DME (2), M = Na; L = Et2O (5), L = DME (6)), [{Li(OdbpOMe)}2] (3) and [{M(OdbpOMe)(L)}2] (M = Li; L = DME (4), M = Na; L = THF (7), L = DME (8)). Complexes 3 and 7 exhibit −OdbpOMe methoxy coordination and all four sodium complexes (5–8) display π–aryl contacts from one phenolate radial arm to each sodium centre. The attempted synthesis of {Na(odbp)}n by direct sodiation of HOdbp yields a small quantity of the 2-benzylphenolate [{Na(Ombp)(DME)}4] (9) (−Ombp = −OC6H4-2-CH2Ph), providing a rare example of benzyl C–C bond scission.

 Please wait while we load your content...
Please wait while we load your content...