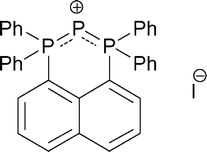
The effect of the special peri-geometry of rigid naphthalene-1,8-diyl backbone in phosphenium formation reaction was investigated. 1,8-Bis(diphenylphosphino) naphthalene and P2I4 afforded triphosphenium iodide in a clean reaction. The reaction of 1,8-bis(dimethylaminophosphino) naphthalene with P2I4 is complex, it afforded four products, all containing the 1,2-dihydro-1,2-diphosphaacenaphthylene motif and heterophosphonium functionalities. Two examples of the rare structural motif of two acenaphthylene units connected head to head and thus a contiguous chain of four phosphorus atoms were also isolated, one compound showing diastereomerization in the solution. All new compounds were fully characterised including single crystal X-ray diffraction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...