Density functional calculations are reported concerning the olefin metathesis characteristics of a variety of P-heterocyclic carbene (PHC) complexes. The calculations employ model catalysts of the type (PMe3)(PHC)Cl2Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, the PHC ligands being 1,3-dihydro-1,3-diphosphol-2-ylidene PH, 1,3-diphenyl-1,3-diphosphol-2-ylidene PPh, and 1,4-dihydro-1,4-diphosphol-2-azol-5-ylidene PNH. Complexes with N-heterocyclic carbenes (NHC) are included for comparison. Associative and dissociative reaction pathways are considered, the latter ones representing the favored reaction mechanisms. Calculations show that the rate determining step is ring opening of a ruthena-cyclobutane intermediate. In comparison with NHC model catalysts, the PHC compounds have lower phosphine dissociation energies, and also form weaker π-complexes with an olefinic substrate. Compared to the initially formed π-complexes, the ruthena-cyclobutane is more stable for PHC- than for NHC-catalysts. The catalytic activity of model PHC-compounds in comparison with NHC-compounds is discussed on the basis of the calculated reaction profiles. In this context, different models for enhanced reactivity of NHC-based catalysts that have been proposed in the literature are considered as well. It is demonstrated that the nature of the substituent of the carbene phosphorus not only exhibits a steric influence on the course of the reaction, but a significant stereoelectronic effect as well. Further, agostic interactions in ruthena-cyclobutane intermediates are investigated.
CH2, the PHC ligands being 1,3-dihydro-1,3-diphosphol-2-ylidene PH, 1,3-diphenyl-1,3-diphosphol-2-ylidene PPh, and 1,4-dihydro-1,4-diphosphol-2-azol-5-ylidene PNH. Complexes with N-heterocyclic carbenes (NHC) are included for comparison. Associative and dissociative reaction pathways are considered, the latter ones representing the favored reaction mechanisms. Calculations show that the rate determining step is ring opening of a ruthena-cyclobutane intermediate. In comparison with NHC model catalysts, the PHC compounds have lower phosphine dissociation energies, and also form weaker π-complexes with an olefinic substrate. Compared to the initially formed π-complexes, the ruthena-cyclobutane is more stable for PHC- than for NHC-catalysts. The catalytic activity of model PHC-compounds in comparison with NHC-compounds is discussed on the basis of the calculated reaction profiles. In this context, different models for enhanced reactivity of NHC-based catalysts that have been proposed in the literature are considered as well. It is demonstrated that the nature of the substituent of the carbene phosphorus not only exhibits a steric influence on the course of the reaction, but a significant stereoelectronic effect as well. Further, agostic interactions in ruthena-cyclobutane intermediates are investigated.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, the PHC
CH2, the PHC 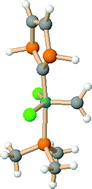

 Please wait while we load your content...
Please wait while we load your content...