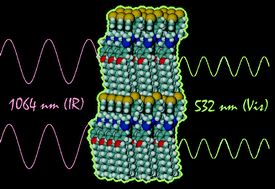
Extraordinary high degrees of polar order can be achieved by a rational design that involves the polar stacking of parallel beloamphiphile monolayers (PBAM). This strategy is exemplified by the acetophenone azines MCA (4-methoxy-4′-chloroacetophenone azine) and DCA (4-decoxy-4′-chloroacetophenone azine). The beloamphiphile design aims to achieve strong lateral interactions by way of arene–arene, azine–azine, arene–azine and halogen-bonding interactions. Dipole-induced interactions and halogen bonding dominate interlayer interactions and halogen bonding is shown to effect the layer stacking. Crystals of DCA contain PBAMs with perfect polar order and perfect polar layer stacking, while crystals of MCA features perfect polar order only in one of two layers and layer stacking is polar but not entirely perfect. We report the synthesis of the beloamphiphile DCA, its crystal structure, and we present a comparative discussion of the structures and intermolecular interactions of MCA and DCA. Absorbance and photoluminescence measurements have been carried out for solutions of DCA and for DCA crystals. DCA exhibits a broad emission centered at 2.5 eV when excited with UV radiation. The nonlinear optical response was studied by measuring second harmonic generation (SHG). Strong SHG signals have been observed due to the polar alignment and the DCA crystal's NLO response is 34 times larger than that of urea. Optimization of the beloamphiphile and systematic SAR studies of the polar organic crystals, which are now possible for the very first time, will further improve the performance of this new class of functional organic materials. The materials are organic semiconductors and show promise as blue emitters, as nonlinear optical materials and as OLED materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...