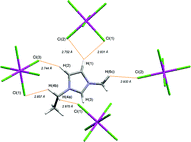
The electrochemistry of the salts, [emim]2[UBr6] and [emim]2[UO2Br4] ([emim] = 1-ethyl-3-methylimidazolium), has been investigated in both a basic and an acidic bromoaluminate(III) ionic liquid. In the basic ionic liquid, the hexabromo salt undergoes a one-electron reversible reduction process at a stationary glassy carbon disc electrode, while the tetrabromodioxo salt was reduced to a uranium(IV) species by an irreversible two-electron process with the simultaneous transfer of oxide to the ionic liquid. On the other hand, dissolution of either of the salts in an acidic bromoaluminate(III) ionic liquid resulted in the formation of the same electroactive species. The solid state structures of the uranium chloride salts, [emim]2[UCl6] and [emim]2[UO2Cl4], have previously been reported, but have now been re-evaluated using a new statistical model developed in our group, to determine the presence or absence of weak hydrogen bonding interactions in the crystalline state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...