Structural diversity in manganese squarate frameworks using N,N-donor chelating/bridging ligands: syntheses, crystal structures and magnetic properties
Abstract
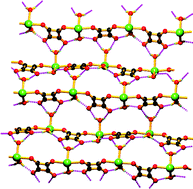
Three new polymeric squarato-bridged manganese complexes {[Mn(H2O)2(bpe)(sq)]·bpe·H2O}n (1), [Mn2(H2O)4(phen)2(sq)2]n (2) and [Mn2(H2O)2(phen)4(sq)]·(sq)·8(H2O) (3) [bpe, 1,2-bis(4-pyridyl)ethane; phen, 1,10-phenanthroline; sq, squarate dianion] have been synthesized and characterized by single crystal X-ray diffraction analysis and variable temperature magnetic studies. Complex 1 is a 2D rectangular grid-like structure, achieved through flexible bpe bridging ligands and squarate dianions. On the other hand the use of chelating phen instead of bpe gives rise to a 1D polymeric chain in complex 2 and to a dinuclear entity in 3. In all the three complexes weak interactions play a vital role in stabilizing the solid-state structure. Variable temperature (2–300 K) magnetic studies indicate weak antiferromagnetic coupling between the metal centres in all the complexes.

 Please wait while we load your content...
Please wait while we load your content...