Fiber-optic infrared reflectance spectroelectrochemical studies of osmium and ruthenium nitrosyl porphyrins containing alkoxide and thiolate ligands†
Abstract
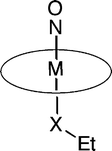
We have examined the redox behavior of the osmium and ruthenium compounds (OEP)M(NO)(OEt) and (OEP)M(NO)(SEt) (OEP = octaethylporphyrinato dianion; M = Os, Ru) by cyclic voltammetry and infrared spectroelectrochemistry. The compound (OEP)Os(NO)(OEt) undergoes a single reversible oxidation process in dichloromethane. In contrast, the thiolate compound (OEP)Os(NO)(SEt) undergoes a net irreversible oxidation resulting in formal loss of the SEt ligand. Extended Hückel calculations on crystal structures of these two compounds provide insight into the nature of their HOMOs. In the case of the alkoxide compound, the HOMO is largely metal centered, with 70% of the charge located in the metal's orbital and ∼25% on the porphyrin ring. However, the HOMO of the thiolate compound consists of a π bonding interaction between the metal dxz orbital and the px orbital on the sulfur, and a π antibonding interaction between the metal d orbital and a π* orbital on NO. The redox behavior of the Ru analogues have been determined, and are compared with those of the Os compounds.

 Please wait while we load your content...
Please wait while we load your content...