Abstract
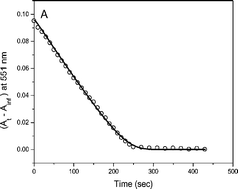
The cobalt(III) complexes, [(NH3)5CoBr]2+ and [(NH3)5CoI]2+ are reduced by Ti(II) solutions containing Ti(IV), generating nearly linear (zero-order) profiles that become curved only during the last few percent of reaction. Other Co(III)–Ti(II) systems exhibit the usual exponential traces with rates proportional to [Co(III)]. Observed kinetics of the biphasic catalyzed Ti(II)–Co(III)Br and Ti(II)–Co(III)I reactions support the reaction sequence:

 Please wait while we load your content...
Please wait while we load your content...