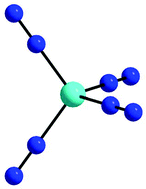
Matrix isolation of Ni atoms in an N2 matrix leads to the formation of Ni(N2)4. This compound, being isoelectronic with the well known Ni(CO)4, represents an important bench-mark system. It has been characterised experimentally by UV/Vis, IR and Raman spectra. The vibrational spectra give evidence for both a1 modes, three of the four t2 modes, and one of the two e modes of the Td symmetric molecule. The experimental data obtained for Ni(14N2)4 and Ni(15N2)4 were used to determine the valence force constants f(Ni–N) and f(N–N), which are compared with those derived for Ni(N2) and for the corresponding carbonyl complexes Ni(CO) and Ni(CO)4. In addition, several overtones and combination modes of Ni(N2)4 were observed for the first time, providing further valuable information about the bond properties. The data allow for the first time a direct estimate of the Ni–N2 bond energy in Ni(N2)4
(120 kJ mol−1), that compares with a value of 148 kJ mol−1 determined by the same method for Ni(CO)4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...