Addition products of a P(iii)-isothiocyanate to dialkyl acetylenedicarboxylates: a spirocyclic phosphinimine and a triphosphorus heterocycle with tetra- and penta-coordinate phosphorus†
Abstract
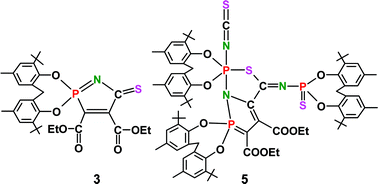
Treatment of the P(III) isothiocyanate CH2[6-t-Bu-4-Me-C6H2O]2PNCS (1) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CO2Et)C(CO2Et)
C(CO2Et)C(CO2Et)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN–}{CH2[6-t-Bu-4-Me-C6H2O]2P(NCS)}–SC
CN–}{CH2[6-t-Bu-4-Me-C6H2O]2P(NCS)}–SC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–P(S)[(OC6H2-6-t-Bu-4-Me)2CH2] with tetra- and penta-coordinate phosphorus, is also isolated. Structure and reactivity of these compounds are discussed. Addition of
N–P(S)[(OC6H2-6-t-Bu-4-Me)2CH2] with tetra- and penta-coordinate phosphorus, is also isolated. Structure and reactivity of these compounds are discussed. Addition of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CO2Et)–C(S)–NH2]
(8) is obtained by evaporating a solution of 7 in open air.
C(CO2Et)–C(S)–NH2]
(8) is obtained by evaporating a solution of 7 in open air.

 Please wait while we load your content...
Please wait while we load your content...