Inside or outside a ligand cleft? Synthetic, structural, and kinetic inertness studies of zinc, cadmium, and mercury complexes of cross-bridged cyclam and cyclen†
Abstract
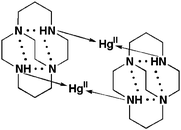
Ethylene cross-bridging of the popular tetraazamacrocyclic ligand cyclam has led to metal complexes with enhanced kinetic inertness. The synthesis and spectral characterization of zinc(II), cadmium(II), and mercury(II) complexes of cross-bridged cyclam (L1) as well as cross-bridged cyclen (L2) are reported along with the details of our synthetic route to L2. X-ray structural studies revealed that all Zn(II) and Cd(II) cations are fully κ4N-coordinated inside the respective ligand's molecular cleft with L1 providing the better fit for Zn(II). While Hg(II) is similarly coordinated to L2, it has been found to complex L1 outside the ligand cleft in a novel exo-κ2N-mode. Solution NMR data of the κ4N complexes are consistent with the presence of only a single cis-folded isomer in each case. Ligand 1H and 13C coupling to both 111,113Cd and 199Hg in their complexes can be clearly discerned. The relative kinetic inertness of representative cross-bridged complexes in acidic aqueous solution has been assessed and found to be in the following order: Zn(II) > Cd(II) ≫ Hg(II). The data also reaffirm that cross-bridged cyclam ligand L1 forms a substantially more inert complex with zinc(II) than either the smaller cyclen analogue L2 or the unbridged 1,4,8,11-tetramethyl–cyclam L3.

 Please wait while we load your content...
Please wait while we load your content...