The reaction of M(CO)3(Ph2PCH2CH2PPh2) (M = Fe, Ru) with parahydrogen: probing the electronic structure of reaction intermediates and the internal rearrangement mechanism for the dihydride products†
Abstract
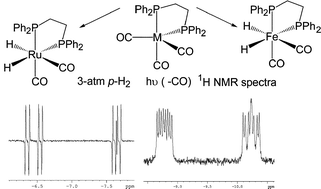
The photochemical reaction of Ru(CO)3(dppe) and Fe(CO)3(dppe) (dppe = Ph2PCH2CH2PPh2) with parahydrogen has been studied by in situ-photochemistry resulting in NMR spectra of Ru(CO)2(dppe)(H)2 that show significant enhancement of the hydride resonances while normal signals are seen in Fe(CO)2(dppe)(H)2. This effect is associated with a singlet electronic state for the key intermediate Ru(CO)2(dppe) while Fe(CO)2(dppe) is a triplet. DFT calculations reveal electronic ground states consistent with this picture. The fluxionality of Ru(CO)2(dppe)(H)2 and Fe(CO)2(dppe)(H)2 has been examined by NMR spectroscopy and rationalised by theoretical methods which show that two pathways for ligand exchange exist. In the first, the phosphorus and carbonyl centres interchange positions while the two hydride ligands are unaffected. A second pathway involving interchange of all three ligand sets was found at slightly higher energy. The H–H distances in the transition states are consistent with metal-bonded dihydrogen ligands. However, no local minimum (intermediate) was found along the rearrangement pathways.

 Please wait while we load your content...
Please wait while we load your content...