Tri(2-thienyl)phosphine (1) has been transformed into chlorotri(2-thienyl)phosphonium chloride (3) in the reaction with hexachloroethane, into tetra(2-thienyl)phosphonium bromide (4) in a NiBr2-catalyzed quaternization with 2-bromothiophene, and into the p-tolylsulfonyliminotri(2-thienyl)phosphorane (6) using “chloramine T”. Attempts to generate the homoleptic penta(2-thienyl)phosphorane (2-C4H3S)5P (5) by treating 3, 4, 6 or the known (PhO)3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2C6H4-2-Me (9) with 2-thienyllithium were unsuccessful. Tri(2-furyl)phosphine (2) was converted into the related imine 7, but the reaction of 7 or of 9 with 2-furyllithium failed to give (2-C4H3O)5P (8). It was only with the strained phosphorane Ph(C12H8)P
NSO2C6H4-2-Me (9) with 2-thienyllithium were unsuccessful. Tri(2-furyl)phosphine (2) was converted into the related imine 7, but the reaction of 7 or of 9 with 2-furyllithium failed to give (2-C4H3O)5P (8). It was only with the strained phosphorane Ph(C12H8)P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2C6H4-4-Me (C12H8
= 2,2′-biphenylylene)
(10) that with 2-C4H3OLi the corresponding phosphorane Ph(C12H8)P(C4H3O-2)2
(11) could be obtained (31P NMR: δ
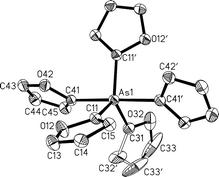
−106.7 ppm). In the arsenic series, tri(2-thienyl)- and tri(2-furyl)arsine (12, 13) were converted into the tosylimino compounds (14, 15) and successfully transformed into the homoleptic arsoranes with 2-C4H3E–Li: penta(2-thienyl)- (16) and penta(2-furyl)-arsorane (17) are stable colourless crystalline solids, the NMR spectra of which indicate rapid pseudo-rotation in solution. The single crystal structure analysis of 17 shows an only slightly distorted trigonal-bipyramidal configuration. In crystals of the phosphine 2 and the arsine 13 the molecules have a propeller configuration with approximate C3v symmetry for the former, but Cs symmetry for the latter. The crystal structures of the precursors or intermediates 3, 4, 6, 9 and 10 have also been determined.
NSO2C6H4-4-Me (C12H8
= 2,2′-biphenylylene)
(10) that with 2-C4H3OLi the corresponding phosphorane Ph(C12H8)P(C4H3O-2)2
(11) could be obtained (31P NMR: δ
−106.7 ppm). In the arsenic series, tri(2-thienyl)- and tri(2-furyl)arsine (12, 13) were converted into the tosylimino compounds (14, 15) and successfully transformed into the homoleptic arsoranes with 2-C4H3E–Li: penta(2-thienyl)- (16) and penta(2-furyl)-arsorane (17) are stable colourless crystalline solids, the NMR spectra of which indicate rapid pseudo-rotation in solution. The single crystal structure analysis of 17 shows an only slightly distorted trigonal-bipyramidal configuration. In crystals of the phosphine 2 and the arsine 13 the molecules have a propeller configuration with approximate C3v symmetry for the former, but Cs symmetry for the latter. The crystal structures of the precursors or intermediates 3, 4, 6, 9 and 10 have also been determined.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2C6H4-2-Me (9) with
NSO2C6H4-2-Me (9) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2C6H4-4-Me (C12H8
= 2,2′-biphenylylene)
(10) that with 2-C4H3OLi the corresponding
NSO2C6H4-4-Me (C12H8
= 2,2′-biphenylylene)
(10) that with 2-C4H3OLi the corresponding 

 Please wait while we load your content...
Please wait while we load your content...