Preparation and topotactical oxidation of ScVO3 with bixbyte structure: a low-temperature route to stabilize the new defect fluorite ScVO3.5 metastable phase
Abstract
ScVO3 has been prepared by controlled ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a
= 9.6182(1)
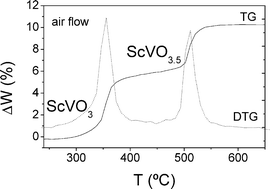
Å. The thermal analysis of ScVO3 in an air flow shows two subsequent
, a
= 9.6182(1)
Å. The thermal analysis of ScVO3 in an air flow shows two subsequent ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a
= 4.947(2)
Å), also showing a random distribution of Sc and V
m, a
= 4.947(2)
Å), also showing a random distribution of Sc and V

 Please wait while we load your content...
Please wait while we load your content...