Synthesis, structure and magnetism of new single molecule magnets composed of MnII2MnIII2 alkoxo-carboxylate bridged clusters capped by triethanolamine ligands†
Abstract
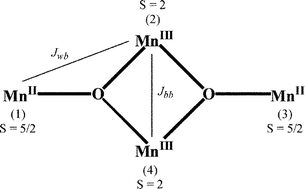
A family of tetranuclear mixed-valent Mn(II)2/Mn(III)2 complexes of type [Mn4(LH2)2(LH)2(H2O)x(RCO2)2](Y)2·nS has been synthesised and structurally characterised, where LH3 = triethanolamine (N(CH2CH2OH)3), {R = CH3, x = 2, Y = CH3CO2−, n = 2, S = H2O; 1}, {R = C6H5, x = 0, Y = C6H5CO2−, n = 1, S = CH3CN; 2}, {R = C2H5, x = 0, Y = ClO4−, n = 0; 3}. A common structural core was deduced from X-ray crystallography and consists of a rhomboidal (planar-diamond) array with two 7-coordinate Mn(II) ‘wingtip (w)’ centres and two 6-coordinate Mn(III) ‘body (b)’ centres. The Mn(III) ions are bridged to the Mn(II) ions by µ3-oxygen atoms from a deprotonated alcohol ‘arm’ of each tridentate LH2− ligand and by µ2-oxygen atoms from each tetradentate LH2− ligand. The four nitrogen atoms from LH2− and LH2− groups, together with bridging and terminal carboxylates oxygens complete the outer coordination sites around the Mn atoms. A feature of these clusters is that they are linked together in the crystal lattice by hydrogen-bonding interactions involving a non-coordinated hydroxyl arm on each LH2− group. Detailed DC and AC magnetic susceptibility measurements and magnetisation isotherms have been made on the three complexes and show that intra-cluster ferromagnetic coupling is occurring between the S = 2 Mn(III) and S = 5/2 Mn(II) ions to yield S = 9 ground states. The g, Jbb and Jwb parameters have been deduced. Inter-cluster antiferromagnetic coupling was noted in 3 and this influences the magnetisation versus field behaviour and the temperature and magnitude of the out-of-phase AC χ″M maxima in comparison to those observed for 1 and 2. An Arrhenius plot of the reciprocal temperature of the maxima in χ″M obtained at different frequencies (10 to 1500 Hz), in the range 1.75 K to 4 K, against the natural logarithm of the magnetization relaxation rate (1/τ) yielded values of the activation energies and pre-exponential factors for two of these new tetranuclear single-molecule magnets (SMMs), 1 and 2. The activation energies were compared with the potential energy barrier height, U, for magnetisation direction reversal (U = DS2) using the axial zero-field splitting parameter, D, deduced from the DC M/H isotherm analysis for these S = 9 species. The very small separation of S = 9 and 8 levels for these clusters highlights the limitations in the determination of D values from M/H data at low temperatures.

 Please wait while we load your content...
Please wait while we load your content...