Gas phase ion chemistry of charged silver(i) adenine ions via multistage mass spectrometry experiments and DFT calculations†‡
Abstract
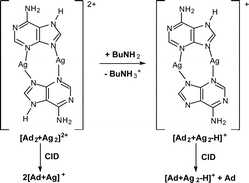
Electrospray ionization (ESI) of solutions containing adenine and AgNO3 yields polymeric [Adx + Agy − zH](y − z)+ species. Density functional theory (DFT) calculations have been used to examine potential structures for several of the smaller ions while multistage mass spectrometry experiments have been used to probe their unimolecular reactivity (via collision-induced dissociation (CID)) and bimolecular reactivity (via ion–molecule reactions with the neutral reagents acetonitrile, methanol, butylamine and pyridine). DFT calculations of neutral adenine tautomers and their silver ion adducts provide insights into the binding modes of adenine. We find that the most stable [Ad + Ag]+ ion does not correspond to the most stable neutral adenine tautomer, consistent with previous studies that have shown that transition metal ions can stabilize rare tautomeric forms of nucleobases. Both the charge and the stoichiometry of the [Adx + Agy − zH](y − z)+ complexes play pivotal roles in directing the types of fragmentation and ion–molecule reactions observed. Thus, [Ad2 + Ag2]2+ is observed to dissociate to [Ad + Ag]+ and to react with butylamine via proton transfer, while [Ad2 + Ag2 − H]+ fragments via loss of neutral adenine to form the [Ad + Ag2 − H]+ ion and does not undergo proton transfer to butylamine. DFT calculations on several isomeric [Ad2 + Ag2]2+ ions suggest that planar centrosymmetric cations, in which two adjacent silver atoms are bridged by two N7H adenine tautomers via N(3),N(9)-bidentate interactions, are the most stable. The [Ad + Ag2 −H]+ ion adds two neutral reagents in ion–molecule reactions, consistent with the presence of two vacant coordination sites. It undergoes a silver atom loss to form the [Ad + Ag − H]+. radical cation, which in turn fragments quite differently to the even electron [Ad + Ag]+ ion. Several other pairs of radical cation/even electron adenine–silver complexes were also found to undergo different fragmentation reactions.

 Please wait while we load your content...
Please wait while we load your content...