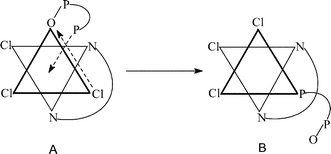
The bimolecular reaction of [ReVL(O)Cl3] [L = 2-(2-pyridyl)benzoxazole (L1), 2-(2-pyridyl)benzthiazole (L2)], 1 with excess diphosphine [Ph2P(CH2)xPPh2
(x
= 1–4)] has furnished [ReIIIL(OP(Ph)2(CH2)xP(Ph)2)Cl3], 2 which is spontaneously converted in solution to [ReIIIL(P(Ph)2(CH2)xP(O)(Ph)2)Cl3], 4. The reaction of [ReIIIL(OPMeyPh3 − y)Cl3], 3 with PMeyPh3 − y (y
= 0–2) has afforded [ReIIIL(PMeyPh3 − y)Cl3], 5. Oxidation of 2 and 3 by dilute nitric acid has furnished nitrates of the rhenium(IV) species, 2+ and 3+. Structure determination vis-à-vis spectral and electrochemical comparisons have revealed a meridional geometry for 2, 3, 2+, and 3+ and a facial geometry for 4 and 5. The transformation 2
→
4 is a twin isomerization (linkage-cum-geometrical), the geometrical part of which recurs in the conversion 3
→
5. Rate studies have revealed that the reaction 2
→
4 is intramolecular in nature. It is initiated by nucleophilic attack of the metal by the dangling phosphine function. The process slows down nearly exponentially as the diphosphine spacer length (x) increases. The oxidised complex 2+ does not isomerize.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...