Diversity of the coordination modes of Croconate Violet. Crystal structures, spectroscopic characterization and redox studies of mono-, di- and poly-nuclear iron(ii) complexes†
Abstract
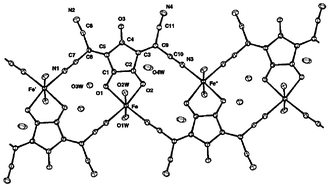
The crystal structures of three iron(II) complexes with Croconate Violet [3,5-bis(dicyanomethylene)cyclopentane-1,2,4-trionate dianion = L2−] have been determined: K2[FeL2(H2O)4]
1, {[Fe(2,2′-bipy)L(H2O)2]·H2O}22 and {[FeL(H2O)2]·2H2O}n3. Complex 1 consists of dianionic pseudo-octahedral mononuclear entities containing two N-mono-coordinated

 Please wait while we load your content...
Please wait while we load your content...