The reaction between excess n-C4H9ONO and arachno-4,5-C2B7H13
(1) in diethyl ether at room temperature results in the formation of the first representatives of the arachno ten-vertex azadicarbaborane family, arachno-6,5,9-NC2B7H12
(2) and arachno-6,5,10-NC2B7H12
(3) in yields of 6 and 20%, respectively. The reaction also generates exo-8-CH3-hypho-7,8-NCB6H11
(4)
(yield 5%) as a result of the extrusion of the CH2 group from the cluster of the starting dicarbaborane to generate a terminal methyl unit. The reaction between 8,9-μ-NH2-arachno-5,6-C2B8H11
(5) and NaH, followed by treatment with iodine and water, produced the unique endo isomer of 4, endo-8-CH3-hypho-7,8-NCB6H11
(6)
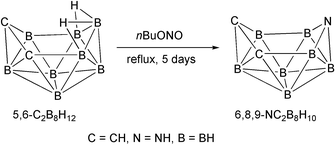
(yield 22%). The reaction between n-C4H9ONO and nido-5,6-C2B8H12
(7) in hexane at reflux generates the first compound of the ten-vertex nido azadicarbaborane family, nido-6,8,9-C2NB7H10
(8), in 13% yield. The structures of all the compounds isolated are proposed on the basis of NMR spectroscopy, the geometries optimized at the RMP2(fc)/6-31G* level and their correctness was supported on the basis of GIAO-SCF NMR calculations.

 Please wait while we load your content...
Please wait while we load your content...