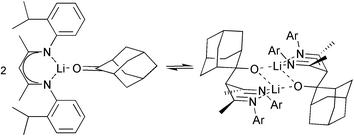
Direct acid-catalysed condensation of substituted anilines with acetylacetone was found to give convenient access to β-enamineimines [2-Pri-C6H4NCMeCHCMeNH-2-Pri-C6H4] and [2-MeO-C6H4NCMeCHCMeNH-2-MeO-C6H4], whereas TiCl4-mediated condensation was required to produce [2,6-Pri2-C6H3NHC(CF3)CHC(CF3)N-2,6-Pri2-C6H3], which was crystallographically characterized. All are conveniently metallated using BunLi. The structures of monomer [2-Pri-C6H4NCMeCHCMeN-2-Pri-C6H4·Li(thf)2], and dimer [{ 2-MeO-C6H4NCMeCHCMeN-2-MeO-C6H4·Li}2] are reported. The structure of the dimeric product of aldol addition of adamantan-2-one to [2-Pri-C6H4NCMeCHCMeN-2-Pri-C6H4·Li.], the lithium scorpionate [{(C10H14OLi)CH(CMeN-2-Pri-C6H4)2}2], is also reported. It undergoes retro-aldol dissociation upon dissolution in non-co-ordinating solvents. The efficient synthesis of α-C,C′ dialkylated ‘true’
β-diimines by repeat lithiation/alkylation of di- and mono-ortho-isopropylanilino diketiminates is also reported. The differing reactivity of the monomers and dimer with electrophiles, and its relation to the structures of the intermediates, are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...