The synthesis, spectroscopic and structural characterization of the bromo-boryl complexes (η5-C5R4R′)Fe(CO)2B(2,4,6-Me3C6H2)Br (R = R′
= H, 2; R = H, R′
= Me, 3; R = R′
= Me, 4) are reported. These are shown to be versatile substrates for the synthesis of both asymmetric boryl complexes [e.g.
(η5-C5H5)Fe(CO)2B(2,4,6-Me3C6H2)OC6H4tBu-4, 6], and bridging borylene complexes {e.g.
[(η5-C5H4R)Fe(CO)2]2B(2,4,6-Me3C6H2), R = H, 7; R = Me, 8}
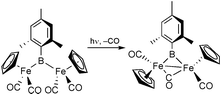
via substitution chemistry with retention of the metal–boron bond. Complexes 7 and 8 are the first reported examples of structurally characterized bridging borylene complexes without a supporting M–M bond. Photolytically induced CO loss from [(η5-C5H5)Fe(CO)2]2B(2,4,6-Me3C6H2) yields the complex [(η5-C5H5)Fe(CO)]2(μ2-CO)[μ2-B(2,4,6-Me3C6H2)] (11), which features a supported bridging borylene ligand.

 Please wait while we load your content...
Please wait while we load your content...