Towards co-operative reactivity in conjoint classical-organometallic heterometallic complexes: the co-ordination chemistry of novel ligands with triphenylphosphine and bis(pyridylethyl)amine or triazacyclononane domains†
Abstract
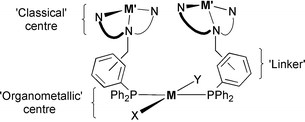
With a view towards later studies of co-operativity in heteronuclear complexes with hard classical (oxygen-activating) and soft organometallic (organic-substrate binding) metal centres, four novel ditopic N3P-donor ligands (L1–L4), each comprising triphenylphosphine tethered to an N,N′-bis(2-pyridyl-2-ethyl)amine (bpea) or a 1,4-diisopropyl-1,4,7-triazacyclononane (tacn*) N3-donor group, have been designed and prepared by reductive aminations of ortho- and meta-(diphenylphosphino)benzaldehydes with bpea (for L1 and L3) and tacn* (for L2 and L4). A range of κNn,κP-chelate mononuclear complexes have been isolated from the reactions of the ortho-substituted ligands, L1 and L2, with Cu(I), Zn(II) and Pt(II) sources, and the X-ray crystal structures of [Cu(L1)][PF6], [Cu(L2)][PF6] (communicated in: S. E. Watkins, D. C. Craig and S. B. Colbran, J. Chem. Soc., Dalton Trans., 1999, 1539) and [PtCl(L1)][PF6] have been determined. Six complexes with the phosphine of L1–L4 co-ordinated to a softer [Pt(II), Ir(I) or W(0)] metal centre and with dangling, metal-free N3-donor domains have been prepared: for the ortho-substituted ligands L1 and L2, it was necessary to protect the hard, more basic N3-donor domains by protonation (pH control) to prevent formation of κNn,κP-chelate mononuclear complexes; for the meta-substituted ligands L3 and L4, pH control was unnecessary as the phosphine group selectively binds to the softer metal ions. The complex trans-[IrCl(CO)(L3)2] reversibly forms a dioxygen adduct. An Ir(III)Cu(II)2 and four Pt(II)Cu(II)2 heterometallic complexes were prepared by adding hard Cu(II) ions to the Ir(I) and Pt(II) complexes with metal-free N3-donor domains, and the full characterisation of these is described. The tungsten(0) carbonyl complex [W(CO)5(L3)], with a metal-free N3-bpea domain, was prepared for a study of metal ion recognition. No perturbation of the carbonyl region of the IR spectrum was observed when metal ions were added. The effect of submolar quantities of heterometallic complexes, obtained by adding a first d-series metal(II) ion (2 equivalents) to [IrCl(CO)(L3)2], on the oxidation of styrene by oxygen in methylethyl ketone has been assayed: inhibition of the oxidation is observed with the %conversion and the product selectivity dependant on the metal(II) ion.

 Please wait while we load your content...
Please wait while we load your content...