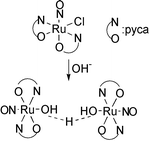
cis-[Ru(NO)Cl(pyca)2] (pyca = 2-pyridinecarboxylate) ([1]), reacts with nucleophiles such as OH− and N3− in H2O to generate the dimeric nitrosylruthenium complex, [{Ru(NO)(pyca)2}2(μ-H3O2)]PF6·2H2O ([2]PF6·2H2O). The bridging unit of [2]PF6 is a hydroxide hydrate anion (H3O2−) composed of a hydroxo and an aqua moiety. Coordinated to each of the ruthenium centers are two pyca ligands in the trans-form with the pyridyl nitrogen atoms and the carboxylic oxygen atoms being at the trans position to each other (trans-form; trans(N,N), trans(O,O)-configuration). [2]PF6 has also been isolated by the reaction of [1] with N3− in H2O. The reaction of [1] with CH3O− in CH3OH gives the trans-form nitrosylruthenium complex, trans-[Ru(NO)(OCH3)(pyca)2]·CH3OH (trans(N,N), trans(O,O)-configuration) ([3]·CH3OH). The rare cis–trans isomerizations have thus occurred during the reaction between [1] (cis-form; trans(N,N), cis(O,O)-configuration) and OH−, N3− or CH3O−.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...