Macrocyclic ligand design. A synthetic, solvent extraction, computational and NMR study of the effect of cryptand flexibility on sodium ion affinity†
Abstract
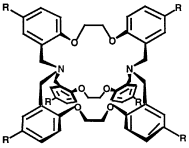
The rigidity of a series of cryptands incorporating an N2O6-donor has been shown to have a marked effect on the ability of such species to complex sodium ions. X-Ray and associated computational investigations, including molecular mechanics, semi-empirical (AM1) and ab initio (density functional) studies, coupled with the results of sodium picrate extraction experiments and NMR studies, have been employed to probe the conformational aspects influencing sodium ion complexation along the ligand series. It is concluded that the ease with which a cryptand is able to adopt an endo–endo conformation is an important factor affecting the occurrence of inclusive metal-ion binding in these systems.

 Please wait while we load your content...
Please wait while we load your content...