Selective photocatalytic oxidation of 3-pyridinemethanol on platinized acid/base modified TiO2†
Abstract
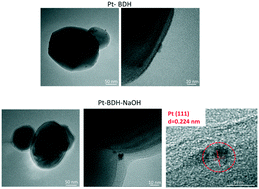
TiO2 catalysts, modified with acidic or alkaline solutions and then platinized, were used for the partial photocatalytic oxidation of 3-pyridinemethanol to 3-pyridinemethanal and vitamin B3 under environmentally friendly conditions. The reaction took place in water under UVA light and air oxygen. Catalysts were characterized by TEM, photoluminescence, DRIFT-IR, Raman, DRS, XPS, and photocurrent measurements. The photocatalytic activity results show that Pt loading of untreated samples leads to a significant activity improvement (hence product yield) as much as acid and alkaline treatments do. Moreover, the alkaline treated TiO2 samples exhibit a further increase in activity after loading with Pt. Pt acts as an electron scavenger promoting electron transfer from the TiO2 conduction band, consequently boosting the photogenerated pair numbers available for the reactive process. Photocurrent measurements show that the TiO2 photocatalysts' active sites increase significantly after platinization and alkaline/acid treatment. The treated and/or Pt loaded catalysts showed good thermal stability (at least up to 400 °C).


 Please wait while we load your content...
Please wait while we load your content...