Modulating the water oxidation catalytic activity of iridium complexes by functionalizing the Cp*-ancillary ligand: hints on the nature of the active species†
Abstract
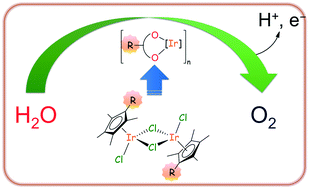
The catalytic activity toward NaIO4 driven water oxidation of a series of [RCp*IrCl(μ-Cl)]2 dimeric precursors, containing tetramethylcyclopentadienyl ligands with a variable R substituent (H, 1; Me, 2; Et, 3; nPr, 4; CH2CH2NH3+, 5; Ph, 6; 4-C6H4F, 7; 4-C6H4OH, 8; Bn, 9), has been evaluated at 298 K and pH = 7 (with phosphate buffer). For each dimer, the effect of changing the catalyst (1–10 μM) and NaIO4 (5–40 mM) concentration has been studied. All precursors exhibit a high activity with TOF values ranging from 101 min−1 to 393 min−1 and TON values being always those expected assuming a 100% yield. The catalytic activity was strongly affected by the nature of the R substituent. The highest TOF values were observed when R was electron-donating and small. The results of multiple consecutive injection experiments suggest that a fragment of the initial C5Me4R, still bearing the R-substituent, remains attached at iridium in the active species, despite the oxidative in situ degradation of the same ligand. The decrease of TOF in the second and third catalytic runs was completely ascribed to a drop of the redox potential caused by the conversion of IO4− into IO3−, according to the Nernst equation. This hypothesis was verified by performing catalytic experiments in which the initial redox potential (ΔE) was deliberately varied by using water solutions of IO4−/IO3− mixtures at different relative concentrations. Consistently, TOF versus ΔE plots show that, for a given catalyst, the same TOF is obtained at a certain redox potential, irrespective of the initial reaction conditions used. All seems to indicate that after a short activation period, during which the transformation of the precursors occurs, individual active species for each dimer form and remain the same also after multiple additions of the sacrificial oxidant. It can be speculated that such active species are small iridium clusters bearing R-functionalized likely O,O-bidentate ligands.


 Please wait while we load your content...
Please wait while we load your content...