Mechanistic understanding of ethane dehydrogenation and aromatization over Zn/ZSM-5: effects of Zn modification and CO2 co-reactant†
Abstract
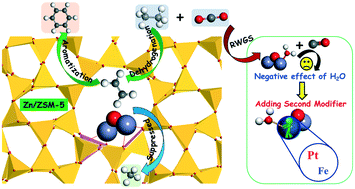
Due to the vigorous development of shale gas production technology, the aromatization of light alkanes has become more attractive for the chemical industry. Ethane dehydrogenation/aromatization over Zn/ZSM-5 catalyst was investigated using density functional theory calculations to clarify the intrinsic effects of introducing a Zn modifier and CO2 co-reactant on the catalytic activity and performance. Introducing Zn to HZSM-5 resulted in the creation of new active sites composed of (Zn–O–Zn)2+ species and thus altered the reaction pathways and reduced the kinetic barriers of ethane dehydrogenation. Moreover, Zn/ZSM-5 significantly suppressed methane by-product formation as compared to the unmodified ZSM-5, leading to an increased selectivity to aromatic products. In the presence of CO2, the H2O produced via the reverse water gas shift (RWGS) reaction could hydrolyze the (Zn–O–Zn)2+ active sites and produce weaker acid sites, which correspond to the increased barriers for ethane dehydrogenation. The participation of H2O in ethane conversion also reduced the catalytic activity of Zn/ZSM-5. The present DFT results predict that adding Pt or Fe as a second modifier for Zn/ZSM-5 helps to prevent the hydrolysis of (Zn–O–Zn)2+ active sites and minimize the negative effect of H2O on ethane conversion, potentially leading to CO2-assisted dehydrogenation/aromatization of light alkanes.


 Please wait while we load your content...
Please wait while we load your content...