New insights into the stereospecific reduction by an (S) specific carbonyl reductase from Candida parapsilosis ATCC 7330: experimental and QM/MM studies†
Abstract
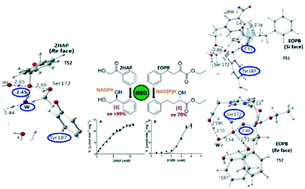
Two representative substrates, a ketone 2-hydroxyacetophenone (2HAP) and an α-ketoester ethyl-2-oxo-4-phenylbutanoate (EOPB) are reduced by a purified (S)-specific carbonyl reductase (SRED) from Candida parapsilosis ATCC 7330 at different rates and kinetic behaviors (hyperbolic vs. sigmoidal), and the resulting alcohols show difference in optical purities. Further, an extensive study employing quantum mechanics/molecular mechanics methods to understand the bases for these differences reveals different reaction coordinates and energy profiles along the reduction paths of 2HAP and EOPB. These complement the experimental observations in which (a) the catalytic efficiency of SRED towards EOPB > 2HAP and (b) the optical purity of the (S)-alcohol is >99% for the reduction of 2HAP while it is ∼70% for EOPB. A plausible explanation for the different reduction mechanisms for 2HAP and EOPB at the atomic level is presented.


 Please wait while we load your content...
Please wait while we load your content...