Temporal control in tritylation reactions through light-driven variation in chloride ion binding catalysis – a proof of concept†
Abstract
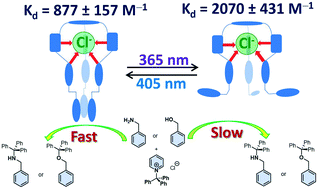
Tripodal triazole-linked azo(hetero)arene-based photoswitchable catalysts T1–5 have been designed, synthesized and optimized for the tritylation reaction of benzylamine (BzNH2). The tritylation reaction rates/yields achieved by light induced isomerization are compared between the native and photoswitched states of the catalyst T1. This concept of controlling the tritylation reaction rates with light has also been extended to additional substrates. The critical role of the triazole C–H⋯Cl− interactions has been confirmed by a combination of spectroscopic, calorimetric and computational studies. Also, the effect of variation in the binding affinities between the native and photoswitched states of the catalyst at room temperature in the temporal control of the catalysis has been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...