Promoting effects of SO42− on a NiMo/γ-Al2O3 hydrodesulfurization catalyst
Abstract
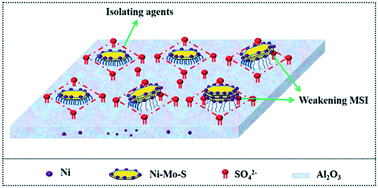
This study details the promoting effects that SO42− has on a NiMo/γ-Al2O3 catalyst prepared using NiSO4 (CAT-Sulf) as a nickel precursor, compared with the Ni(NO3)2 (CAT-Nit) and the industrial NiMoP-complex (CAT-NiP) after 600 °C calcination. The three catalysts were characterized by virtue of X-ray diffraction (XRD), thermogravimetry mass spectrometry (TG-MS), N2 adsorption–desorption, hydrogen temperature programmed reduction (H2-TPR), X-ray photoelectron spectroscopy (XPS) and high-resolution transmission electron microscopy (HRTEM). Hydrodesulfurization (HDS) activity was assessed using dibenzothiophene (DBT) as the feedstock. On the one hand, compared with CAT-Nit, the SO42− anions could be anchored on the catalyst surface due to their thermal stability and strong interaction with Al2O3, while few NO3− anions could be found. These anchored SO42− anions weaken the metal–support interaction and hinder the formation of NiAl2O4, thereby facilitating both the sulfidation of Mo species and the decoration of Ni atoms and generating more Ni–Mo–S active sites. On the other hand, compared with CAT-NiP, the residual SO42− anions do not impair the support properties and CAT-Sulf maintains excellent textural structure, i.e. high specific surface area and a proper pore structure. Therefore, CAT-Sulf exhibits the highest dibenzothiophene hydrodesulfurization (DBT-HDS) activity out of the three catalysts. Moreover, these anchored SO42− anions effectively hinder the aggregation and growth of the active phases, thus guaranteeing a catalyst with high metal dispersion and good stability. Finally, using the inferior residual oil as a feedstock, the stability of CAT-Sulf was also assessed compared with an industrial catalyst (CAT-Ref), and the results show that during 1500 h on stream, its HDS ratio is maintained at around 78%, while that of the industrial catalyst (CAT-Ref) declined from ca. 82% to ca. 71% after 1200 h, indicating that CAT-Sulf exhibits much better stability and shows excellent industrial potential. The anchored SO42− anions simultaneously endow the NiSO4-derived catalyst with excellent physical structure, high intrinsic activity and good stability, indicating that it might be a simple and effective method to balance the activity and stability of the HDS catalyst using thermostable nickel precursor salts.


 Please wait while we load your content...
Please wait while we load your content...