Manipulating the regioselectivity of a Solanum lycopersicum epoxide hydrolase for the enantioconvergent synthesis of enantiopure alkane- and alkene-1,2-diols†
Abstract
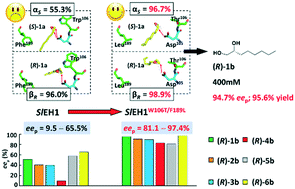
The hydrolysis of epoxides by epoxide hydrolases (EHs) is a sustainable approach to synthesize chiral vicinal diols. However, few EHs hitherto reported can catalyse the enantioconvergent synthesis of enantiopure alkane- and alkene-1,2-diols, due to the strict desire for regiocomplementarity. To actualize the enantioconvergent hydrolysis of rac-1,2-epoxyoctane (1a), the regioselectivity (αS, 55.3%) of SlEH1 for (S)-1a was manipulated via site-directed mutagenesis. Ten specific residues lining the substrate-binding pocket of SlEH1 were identified based on the computer-aided design, each of which was replaced by seven residues, respectively. Among 66 single-site mutants, SlEH1W106L, SlEH1W106T, SlEH1F109I, SlEH1M180V, SlEH1F189I and SlEH1F189L were selected, by which rac-1a was hydrolysed into (R)-octane-1,2-diol (1b) with an eep range of 62.1–82.4%. After combinatorial mutagenesis and screening, one double-site mutant, SlEH1W106T/F189L, was obtained having the highest αS of 96.7%. The gram-scale enantioconvergent hydrolysis of 400 mM (51.3 g L−1) rac-1a was carried out using 200 mg mL−1 wet cells of E. coli/sleh1W106T/F189L at 20 °C for 24 h, producing (R)-1b with 94.7% eep and 95.6% yield. The substrate spectrum assay of SlEH1W106T/F189L towards 20 mM rac-1a–6a was conducted, producing (R)-1b–6b with 81.1–97.4% eep values. Furthermore, the molecular dynamics simulation analysis indicated that the regiopreference of SlEH1W106T/F189L attacking on the Cα of (S)-1a was greater than that of SlEH1, which was consistent with their αS values measured experimentally. This work engineered a superior double-site mutant, SlEH1W106T/F189L, for the enantioconvergent synthesis of a variety of chiral alkane- and alkene-1,2-diols, especially (R)-1b and 6b, with high eep values.


 Please wait while we load your content...
Please wait while we load your content...