Substrate hydrophobicity and enzyme modifiers play a major role in the activity of lipase from Thermomyces lanuginosus†
Abstract
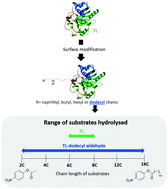
Lipase from Thermomyces lanuginosus (TL) displays high affinity for long-chain substrates, such as triolein and other long-chain triacylglycerols. Aiming to broaden the substrate chain-length specificity, different aldehydes (naphthaldehyde, butyraldehyde, hexyl aldehyde and dodecyl aldehyde) and naphthyl isothiocyanate were grafted onto lipase TL through lysine coupling. The catalytic activity of the modified lipases was investigated by reaction with substrates differing in the aliphatic chain size (p-nitrophenyl benzoate, p-nitrophenyl acetate, p-nitrophenyl butyrate, p-nitrophenyl hexanoate, p-nitrophenyl octanoate, p-nitrophenyl laurate and p-nitrophenyl palmitate). The enzymes modified with aldehydes revealed higher activity than the enzymes modified with the isothiocyanate. The most notable results were achieved for lipase TL grafted with 4 units of a dodecyl chain (TL5), which revealed the highest activity against all the tested substrates, being 10-fold more active than the native enzyme for smaller substrates (acetate and butyrate chains) and 2-fold for longer substrates (laurate and palmitate chains). The kinetic parameters evaluated (Vmax, KM and kcat/KM) also confirmed the significant catalytic performance of TL5 compared to the native enzyme. The increase in activity revealed by the modified lipases was directly proportional to the size and hydrophobicity of the linkers' aliphatic chain. Small conformational changes, either on the enzyme's lid or on the cavity of the active site were suggested by molecular dynamics simulations, circular dichroism and fluorescence spectroscopy. Moreover, the grafting with aldehydes or with the isothiocyanate conferred higher thermostability to the lipase. The chemical surface modification developed efficiently improved the activity of lipase TL, broadening the substrate's chain-length specificity, increasing thereafter the substrate possibilities for industrial reactions.


 Please wait while we load your content...
Please wait while we load your content...