Understanding the co-effects of manganese and cobalt on the enhanced SCR performance for MnxCo1−xCr2O4 spinel-type catalysts†
Abstract
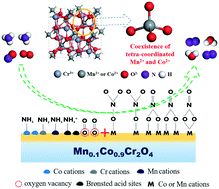
A novel ternary Mn0.1Co0.9Cr2O4 catalyst was identified from a range of chromium-based spinel-type (XCr2O4) oxides for the SCR of NOx in coking flue gas. It exhibited a broad operating window (166–393 °C) under a gas hourly space velocity of 112 000 h−1 with excellent H2O and SO2 durability. Compared to MnCr2O4 and CoCr2O4, the coexistence of Mn and Co cations in Mn0.1Co0.9Cr2O4 resulted in the enrichment of favorable Co3+, Mn4+, and Cr5+ species and oxygen vacancies, and enhanced Lewis acidity and redox ability. Mn and Co cations stably exist on the A2+ sites of MnxCo1−xCr2O4 when x is less than 0.2, and the spinel-type structure preservation was conducive to their increased SCR activity. In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) revealed that both CoCr2O4 and Mn0.1Co0.9Cr2O4 followed the Langmuir–Hinshelwood mechanism, while higher amounts of adsorbed ammonia and nitrate species were produced on Mn0.1Co0.9Cr2O4, and the reaction rates were much higher. In addition, DFT demonstrated that the oxygen vacancy formation energy was lowered by Mn doping, and the enhanced affinity of NH3 and NO with Mn0.1Co0.9Cr2O4 helps to form conducive NH2 and nitrate species which facilitate the SCR reaction.


 Please wait while we load your content...
Please wait while we load your content...