A deactivation mechanism study of phosphorus-poisoned diesel oxidation catalysts: model and supplier catalysts
Abstract
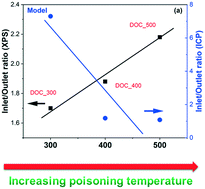
The effect of phosphorus poisoning on the catalytic behavior of diesel oxidation catalysts was investigated over model and supplier monolith catalysts, i.e., Pd–Pt/Al2O3. The results of ICP and XPS from the vapor-phase poisoning over model catalysts suggested that the temperature of phosphorus poisoning affects both the overall content of phosphorus and the dispersion of phosphorus (i.e., inlet/outlet and surface/bulk). Phosphorus oxide (P2O5), metaphosphate (PO3−), and phosphate (PO43−) were identified in the poisoned model and supplier catalysts. The distribution of these species on poisoned model catalysts was highly dependent on the poisoning temperature, i.e., a higher temperature resulted in a higher concentration of PO43−. The outlets of the monoliths contained more PO43− and less P2O5 than the inlets. Both active sites and surface OH groups on model and supplier catalysts were contaminated upon phosphorus poisoning. It is found that PO43− had a stronger influence on the active sites than P2O5. One significant finding in this study is that the vapor-phase phosphorus poisoning could be a practical and cost efficient approach to simulate an accelerated aging/poisoning process.


 Please wait while we load your content...
Please wait while we load your content...