Binuclear O2 activation and hydrogen transfer mechanism for aerobic oxidation of alcohols†
Abstract
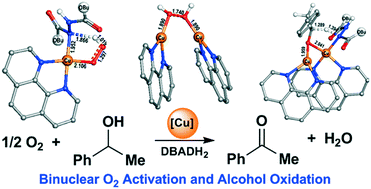
A density functional theory study of the aerobic oxidation of 1-phenylethanol into acetophenone catalysed by phenanthroline copper (phenCu) complexes reveals a binuclear O2 activation and hydrogen transfer mechanism with multiple spin-crossover steps. When di-tert-butyl azodicarboxylate (DBAD) exists, it acts as a stoichiometric oxidant and forms DBADH2 through successive transfers of the proton and hydride from 1-phenylethanol to DBAD in one transition state with a free energy barrier of 21.8 kcal mol−1. After the consumption of DBAD, DBADH2 acts as a co-catalyst assisting O2 activation and acetophenone formation through binuclear transition states for the cleavages of O–O and C–H bonds with a total free energy barrier of 24.6 kcal mol−1. Without the presence of DBAD or DBADH2, the total free energy barrier for the aerobic oxidation of 1-phenylethanol with the participation of two phenCu complexes is 26.1 kcal mol−1. In all the above three situations, the rate-determining step is the activation of the C–H bond in 1-phenylethanol. The formation of HOO˙ radical and the breaking of the O–O bond in hydrogen peroxide for the formation of a Cu(II)-hydroxyl dimer are also key steps in the reaction and need the participation of two phenCu complexes.


 Please wait while we load your content...
Please wait while we load your content...