H/D isotopic exchange between methane and a proton-conducting oxide: theory and experiment†
Abstract
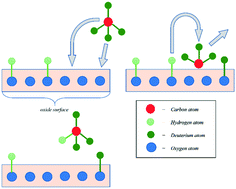
A mechanistic approach taking into account hydrogen incorporation into an oxide structure has been developed for the study of methane activation kinetics and the mechanism on a proton-conducting oxide. Five types of exchange have been proposed, with each type distinguishable with respect to the amount of exchangeable hydrogen in the elementary reaction step. The methane activation process has been investigated with proton-conducting oxide La0.95Sr0.05ScO3−δ powder as a catalyst. H/D isotopic exchange experiments were carried out using a 10 mbar total pressure 9 : 1 mixture of methane/hydrogen in the range of 673–1073 K to analyse the methane activation mechanism on La0.95Sr0.05ScO3−δ. Methane activation kinetics have been considered within the framework of the proposed two-step mechanism. The as-proposed mechanism includes two sequential steps: the dissociative adsorption of methane, forming CHx-type particles and surface OH-groups; and hydrogen incorporation into the oxide structure.


 Please wait while we load your content...
Please wait while we load your content...