Fine-tuning of the substrate binding mode to enhance the catalytic efficiency of an ortho-haloacetophenone-specific carbonyl reductase†
Abstract
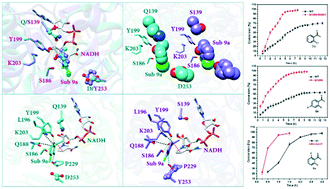
Carbonyl reductase BaSDR1 has been identified as a potential ortho-haloacetophenone-specific biocatalyst for the synthesis of chiral 1-(2-halophenyl)ethanols due to its excellent stereoselectivity. However, the catalytic efficiency of BaSDR1 is far below the required level for practical applications. Thus, fine-tuning of the substrate binding mode, which aimed at maximum preservation of the positive factors for substrate specificity and stereoselectivity, was proposed as a tentative strategy for enhancing its catalytic efficiency. The designed mutants Q139S, D253Y and Q139S/D253Y showed significantly enhanced catalytic efficiency. Remarkably, the variants Q139S and Q139S/D253Y exhibited a more than 9-fold improvement in catalytic efficiency (kcat/Km) toward substrates 6a and 11a, respectively. More importantly, none of the variants caused activity–stereoselectivity trade-off and all variants exhibited excellent stereoselectivity (99% ee). Analysis of variant–substrate complexes showed that the mutations indeed enable the fine-tuning of the substrate binding mode. New strengthening factors for consolidating the productive conformation were introduced while the original positive factors were preserved. Furthermore, at a substrate concentration of 100 mM, recombinant E. coli whole cells expressing the BaSDR1 mutants were successfully applied to the synthesis of several key intermediates of chiral pharmaceuticals, including (S)-1-(2-chlorophenyl)ethanol, (S)-1-(2,4-difluorophenyl)ethanol and (S)-1-(2,6-difluorophenyl)ethanol, with 99% enantiomeric excess, and the conversion reached over 95% in a certain period of time. These results demonstrated the effectiveness of the strategy involving the fine-tuning of the substrate binding mode and the applicability of the designed mutants in efficient reduction of ortho-haloacetophenones.


 Please wait while we load your content...
Please wait while we load your content...