Deactivation of Co-Schiff base catalysts in the oxidation of para-substituted lignin models for the production of benzoquinones†
Abstract
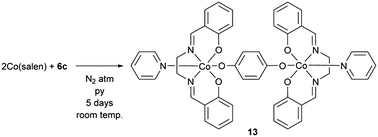
The effect of quinones on the deactivation of four- and five-coordinate Co-Schiff base catalysts used for the oxidation of lignin models is systematically studied. 2,6-Dimethoxy-1,4-benzoquinone does not affect the catalytic activity of any of the studied Co-Schiff base catalysts, but 1,4-benzoquinone and 2-methoxy-1,4-benzoquinone have a strong effect on the catalytic activity. Quinone solubility in the reaction solvent does not correlate with catalyst deactivation, but added pyridine (a basic axial ligand) promotes catalyst deactivation by quinone. The synthesis and characterization of a catalytically inactive Co-Schiff base-quinone complex is presented and preliminary computational analysis of this complex in comparison to a dimeric Co-Schiff base peroxo complex is also discussed. Quinone and the Co-Schiff base redox potentials are found to correlate with catalyst deactivation. Thus, catalysts with a lower redox potential were more susceptible to deactivation, and quinones with a higher redox potential deactivate the catalysts. Based on these results, two mechanisms for deactivation of the catalyst are proposed. The first mechanism describes how the formation a Co-Schiff base-quinone complex prevents formation of the key catalytically active Co-superoxo complex. The second proposed mechanism suggests that quinones inhibit the Co-Schiff base catalyst by scavenging intermediate Co-superoxo radicals.


 Please wait while we load your content...
Please wait while we load your content...
