Selective mild oxidation of methane to methanol or formic acid on Fe–MOR catalysts†
Abstract
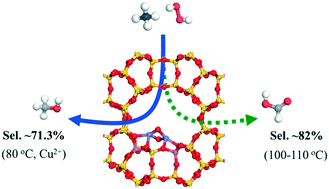
Controllable methane oxidation directly into value-added products under mild conditions remains a challenge. Herein, an active Fe/MOR catalyst was synthesized via simple solid-state ion exchange, and its activity in the selective oxidation of methane with H2O2 in the aqueous phase was intensively investigated. The octahedral dimeric Fe3+ species [Fe2(μ-O)2] in the extra framework was confirmed as the initial active site by X-ray photoelectron spectroscopy, X-ray absorption near-edge structure and extended X-ray absorption fine structure, UV-vis diffuse-reflectance spectroscopy, and high-angle annular dark-field scanning transmission electron microscopy in combination with DFT calculations. The DFT calculations indicated that methanol formation via methyl peroxide (CH3OOH*) on [Fe2(μ-OH)2O2] is the most favorable pathway compared to the direct formation of methanol via CH3O*. The formed CH3OH is easily further oxidized by hydroxyl radicals (˙OH) resulting in non-selective methane oxidation. In contrast, the Fe/MOR catalyst could lead to a high methanol selectivity of 71.3% in the presence of homogeneous Cu2+ precursor, which efficiently suppressed the over-oxidation of methanol, and a high formic acid selectivity up to 81–82% at a slightly higher reaction temperature by mildly shifting the oxidation of methanol and formaldehyde to the target product.


 Please wait while we load your content...
Please wait while we load your content...