Why does tautomycetin thioesterase prefer hydrolysis to macrocyclization? Theoretical study on its catalytic mechanism†
Abstract
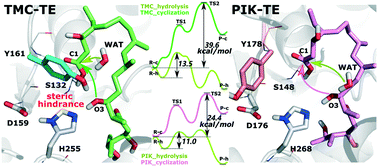
Tautomycetin (TMC) is a naturally linear polyketide metabolite with a variety of pharmacological activities. An experiment, swapping of the TMC thioesterase (TE) domain with pikromycin (PIK) TE, indicates that not only a linear TMC precursor, but also a cyclized TMC was produced. In this study, we attempt to uncover the reasons why TMC TE prefers hydrolysis rather than macrocyclization, and reveal the molecular basis of TE-catalyzed hydrolysis and macrocyclization. Two systems (TMC-TE and PIK-TE systems) were constructed and both MD simulations and QM/MM methods were combined to investigate the catalytic mechanism of the TEs. Pre-reaction states (PRSs), which are critical to macrocyclization, were observed in both systems. Residue Y161 in TMC TE was noticed by analyzing hydrogen bonding and hydrophobic interactions, which seemed to inhibit the effective deprotonation of the substrate, and block the nucleophilic attack owing to steric hindrance. The additional simulations indicated that the Y161A mutant might improve the cyclization by increasing the proportion of PRSs. Besides, more water molecules appeared around the active site of the TMC-TE system, which might improve the probability of hydrolysis. Additionally, the QM/MM calculations indicated that compared to macrocyclization, hydrolysis readily takes place. The barrier for hydrolysis was calculated to be 13.5 and 11.0 kcal mol−1 in TMC-TE and PIK-TE systems, respectively. Besides, macrocyclization may hardly occur in TMC TE (39.6 kcal mol−1), while it may slightly proceed in PIK TE (24.4 kcal mol−1). Our study elucidates the molecular basis of the catalytic mechanism of TMC TE and PIK TE and furthers the discovery and design of novel engineered macrolactones.


 Please wait while we load your content...
Please wait while we load your content...