Electrochemical tuning of heterojunctions in TiO2 nanotubes for efficient solar water splitting
Abstract
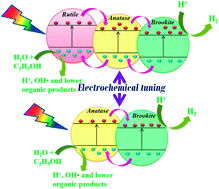
Biphase ↔ triphase switching in TiO2 nanotubes is observed with the change in electrolyte concentration using the rapid breakdown anodization technique. The phase composition of TiO2 nanotubes switches from anatase–rutile–brookite to anatase–brookite and vice versa. The tubular morphology of the samples is revealed from the SEM and TEM micrographs. All the phases are found to be present in a single nanotube, forming heterojunctions. The photocatalytic hydrogen generation efficiencies of TiO2 nanotubes with anatase–rutile–brookite heterojunctions are compared with those of nanotubes having anatase–brookite (obtained by tuning the electrolyte concentration) and anatase–rutile heterojunctions (obtained from the same technique reported elsewhere). The higher water splitting efficiencies of triphasic heterojunctions compared to those of biphasic junctions are attributed to effective charge separation because of cascaded charge transfer through sequential heterojunctions. This leads to the accumulation of electrons and holes in the lowest conduction band and highest valence band levels, respectively, among the phases. The present study supports the theory that a triphasic system is more efficient in photocatalysis compared to two-phase systems. In addition, this study also reports that anatase–brookite biphasic TiO2 is more efficient compared to the widely studied anatase–rutile biphasic TiO2 for the first time.


 Please wait while we load your content...
Please wait while we load your content...