Cyclic telluride reagents with remarkable glutathione peroxidase-like activity for purification-free synthesis of highly pure organodisulfides†
Abstract
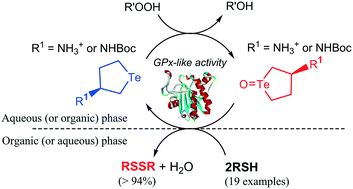
Monoamino cyclic tellurides with a five- or six-membered ring structure and their derivatives were developed as a new class of catalyst for the oxidation of organothiols to organodisulfides in a glutathione peroxidase-like catalytic reaction. Quantitative conversion and high reaction rate were achieved by performing the reaction in an organic–aqueous segmented microflow system. Importantly, the process circumvented product purification, which is a major limitation of current organodisulfide synthetic methods.


 Please wait while we load your content...
Please wait while we load your content...