Novozym 435: the “perfect” lipase immobilized biocatalyst?
Abstract
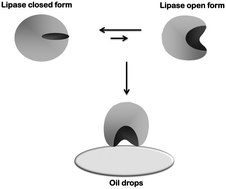
Novozym 435 (N435) is a commercially available immobilized lipase produced by Novozymes. It is based on immobilization via interfacial activation of lipase B from Candida antarctica on a resin, Lewatit VP OC 1600. This resin is a macroporous support formed by poly(methyl methacrylate) crosslinked with divinylbenzene. N435 is perhaps the most widely used commercial biocatalyst in both academy and industry. Here, we review some of the success stories of N435 (in chemistry, energy and lipid manipulation), but we focus on some of the problems that the use of this biocatalyst may generate. Some of these problems are just based on the mechanism of immobilization (interfacial activation) that may facilitate enzyme desorption under certain conditions. Other problems are specific to the support: mechanical fragility, moderate hydrophilicity that permits the accumulation of hydrophilic compounds (e.g., water or glycerin) and the most critical one, support dissolution in some organic media. Finally, some solutions (N435 coating with silicone, enzyme physical or chemical crosslinking, and use of alternative supports) are proposed. However, the N435 history, even with these problems, may continue in the coming future due to its very good properties if some simpler alternative biocatalysts are not developed.


 Please wait while we load your content...
Please wait while we load your content...