The impact of lignin sulfonation on its reactivity with laccase and laccase/HBT†
Abstract
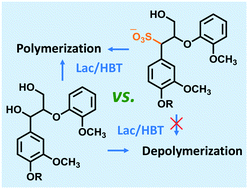
Lignin is a highly abundant aromatic polymer in nature, but its controlled cleavage or cross-linking is a major challenge and currently hindering industrial applicability. Laccase (L) and laccase/mediator systems (LMS) are promising tools for enzymatic lignin modification, but to date, their overall reaction outcome is hard to predict and control. This research aimed to understand the reactivity of native and sulfonated β-O-4 linked lignin structures in L and LMS treatments. Trametes versicolor laccase, and the mediator hydroxybenzotriazole (HBT) were used, and reaction products were analyzed using UHPLC-MSn and MALDI-TOF-MS. Polymerization was observed for both the native and sulfonated phenolic compounds, suggesting that sulfonation does not affect radical coupling of the phenolic lignin subunits. In contrast, sulfonation of the non-phenolic lignin structure prevented Cα oxidation and cleavage by L/HBT, which was explained by an increased Cα–H bond dissociation energy of ∼10 kcal mol−1 upon sulfonation. Overall, our results indicate that lignin sulfonation drives the overall outcome of LMS incubations towards polymerization.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...